sterility test for eye drops|microbiology of eye drops : services All ophthalmic products must meet regulatory standards for microbiological quality and safety for their intended use. This chapter outlines holistic control strategies for sterility, endotoxin, and preservative efficacy to achieve ophthalmic product safety. web11 de fev. de 2024 · 2. YT Saver: A Worthy Alternative. As an alternative to SnapDownloader, YT Saver stands tall with its ability to download videos, audio, and playlists from an impressive array of over 10,000 websites, including Pornhub and several other adult websites. This expansive reach makes YT Saver an attractive option for users .
{plog:ftitle_list}
Resultado da Click on the login button in the top right-hand corner; Enter your email or phone and password; Click the login button at the bottom of the form. If you have forgotten your password, you can click on the forgot password link beneath the login form. This will open a new screen and allow you to enter .
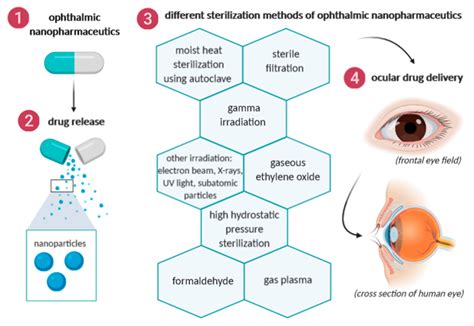
All ophthalmic products must meet regulatory standards for microbiological quality and safety for their intended use. This chapter outlines holistic control strategies for sterility, endotoxin, and preservative efficacy to achieve ophthalmic product safety.We would like to show you a description here but the site won’t allow us.Common to all ophthalmic dosage forms is the critical requirement for sterility of the finished product as well as consideration of the sensitivity of ocular tissue to irritation ( 7 ). 3.1 Solutions
Preservatives in eye drops, while not always necessary, can lead to undesirable effects. Developing preservative-free solutions demands special measures for sterility, .
sterility of ophthalmic drugs
of sterile 70 drug products must comply with CGMP requirements to ensure product sterility. Sterility Test of Eye Drops The sterility of the eye drops was tested following the requirements of the European Pharmacopoeia 10th Edition (2019; chapter 2.6.1. Sterility) [ .ASHP Guidelines on Pharmacy-Prepared Ophthalmic Products. Pharmacists are frequently called on to prepare sterile prod-ucts intended for ophthalmic administration when a suitable sterile . The test involves the touching of an anesthetized cornea by a tonometer tip that aims to record the amount of force required to flatten the cornea. To implement sanitization of .
One critical aspect of quality control is the rigorous testing of eye drop products for sterility (USP <71>). Considering the recent eye drop product recalls involving contamination with drug-resistant Pseudomonas aeruginosa, . The present study aimed to develop clear aqueous rebamipide (REB) eye drops to enhance solubility, stability, patient compliance, and bioavailability.
An ophthalmic product needs to comply with a validated sterility test (European Pharmacopeia 2019a; United States Pharmacopeia and National Formulary (USP 41-NF 36) 2018b; . Introduction. The ophthalmic drug market, valued at .2 billion, plays a significant role in driving growth within the healthcare industry ().Eye drops account for 89% of all registered ophthalmic drugs ().This is largely due to their non-invasive nature and ease of access to various segments of the eye, making them a preferred method of treatment for ophthalmic .
Objectives: To assess microbial contamination of common non-preservative eye drops stored at 4 degrees C and non-preservative fortified antibiotic eye drops used in a hospital inpatient setting. Material and method: A prospective study of the sterility of non-preservative eye drops was examined by dividing the patients into 2 groups. Group 1 . Purpose: When commercial atropine eye drops are not available, diluted atropine eye drops are used for myopia control. However, long-term stability and sterility of self-prepared 0.01% atropine .
Discover the best eye drops for dry eyes, according to a panel of medical experts. Compare our top-rated picks to treat dry eye here. A topical eye drop is the most convenient and patient compliant route of drug . The test involves the touching of an anesthetized cornea by a tonometer tip that aims to record the amount of force required to flatten the cornea. . known as complex systems, which are made of multiple components, should necessarily be sterile [11,12,13].When preservative-free artificial tear solutions are not adequate to reduce symptons, the patient's own serum can be compounded into eye drops that improve the ocular surface. The aim of our study was to test the sterility of autologous serum eye drops in refrigerator conditions for up to 30 days and in freezer conditions for up to 180 days.
Artificial tears can be helpful, but they have risks you should know. Learn how to find safe eye drops, plus alternative remedies for dry eye. . federal law does not require premarket approval for OTC eye drops. But it does require eye drops to be sterile for safe use. “The FDA reminds manufacturers, distributors, repackagers, relabelers .
Download scientific diagram | Sterility test results of eye drops from publication: EFFECT OF STERILIZATION BY HEATING IN THE PRESENCE OF BACTERICIDE AND BACTERIAL FILTERED MEMBRANE ON THE . Eye drops are sterile preparations intended for instillation into the eye. All major pharmacopoeias require these products to pass the antimicrobial effectiveness test (AET). Eye drops, when contaminated with microorganisms, can introduce harmful bacteria, fungi, or viruses into the eye, leading to serious eye infections and potential vision loss. Testing for sterility acts as a safeguard against these risks, ensuring that the products are free from harmful pathogens and safe for use before being released to consumers.
ABSTRACT: Eye drops are sterile preparations intended for instillation into the eye. All major pharmacopoeias require these products to pass the antimicrobial effectiveness test (AET).The result of eye drops being contaminated with Pseudomonas aeruginosa can be a considerable loss of vision or even complete blindness, as happened to some . ypical sterility test procedure performed in an isolator 041-043clt0915Sartorius amended.qxp_Layout 1 01/09/2015 11:26 Page 41. 42 September 2015content, sterility test besides permissible preservatives and packing specifications etc. . Passes Liberman Burchard test Extract 10 ml. eye drops in vol. of extract to one ml. add1.2.1 A failure mode and effects analysis, or equivalent, should be performed with respect to the container consistently delivering eye drops of appropriate microbiological quality, throughout the in-use shelf-life, including consideration of inappropriate use and storage or damaged product (e.g. drop test and testing in a range of appropriate .
Stability eye drop in accelerated and long-term test conditions. Sterile eye drop solutions in bottles and minims were stored in a climate chamber under accelerated test (40°C/75% RH; RH – relative humidity) and long-term test (25°C/60% RH) as well as in a refrigerator under uncontrolled conditions (2-8°C).
Eye drops are sterile preparations intended for instillation into the eye. All major pharmacopoeias require these products to pass the antimicrobial effectiveness test (AET). This test is similar to that used for oral dosage form despite the fact that both product categories differ in their microbiological specifications. The eye drops might pass the official requirements of .
Sterility test. Based on observations, . The serum eye drop is typically well tolerated and can be one of the best treatment options for severe DED due to its close resemblance to tears .Eye drops and other ophthalmic preparations are used by millions of individuals all over the world; in order to keep them safe and guarantee effectiveness, maintaining sterility is vital to proper ophthalmic manufacturing. Ophthalmic manufacturing; that is, the manufacturing of any preparations or applications intended to be used directly on the conjunctiva or conjunctival sac . Based on the evaluation results, all autologous serum eye drops that were formulated met the requirement of eye drops preparations stated in the Indonesia Pharmacopoeia. The preparations are isotonic, clear, sterile, and free from endotoxins. The addition of 25 μg/mL hEGF to the eye drops increased cell viability by up to 197%.
gas spring life test manufacturer
gas spring life test supplier
EYE DROPS: Eye drops are sterile, aqueous or oily solutions or suspensions of one or more medicaments intended for instillation into the conjunctival sac. . Test procedure for eye drops. Uniformity of Volume. This complies with the tests for contents of packaged dosage forms. Particle size. This test is applicable only to eye drops that are . Eye drops are available by prescription or sold as OTCs. . Any drug used in the eyes must be sterile to reduce the risk of infection. Eye drops are available by prescription or sold as OTCs.
To validate the sterility applicability test, microbial growth clearly observable and visually comparable to that observed without product was observed each day for 5 days. To assess the sterility test of eye drops ataluren oily solution, the same procedure was applied, and the potential microbial growth was observed each day for 14 days.
In patients with DED, treatment with 20% AS eye drops significantly improves tear production, lachrymal flow and stability tests and the findings of CIC with transfer. The proposed treatment for DED patients based on 20% AS eye drops appears to be very promising with good results. The improvements are more significant in patients with severe DED.Ophthalmic Preparations—Quality Tests 771 and will include descriptions of and quality . Topical administration is employed mostly in the form of eye drops, ointments, gels, or . Common to all ophthalmic dosage forms is the critical requirement for sterility of the finished
Results. The sterility test was validated with the R2A medium for our ophthalmic preparations. The elimination of the inhibitory effect was achieved using a washing cycle of 500 mL of sodium chloride for ceftazidim, amikacin, cefuroxim, cyclosporine, and vancomycin preparations.Pilocarpine required the use of 500 mL of sterile water for irrigation and 90 mL of .as eye cups, eye droppers, and other dispensers intended for ophthalmic use must also be sterile (Code of Federal Regulations 2018a; European Pharmacopeia 2019a). . While sterility tests are required by regulation for sterile products (Code of Federal Regulations 2018e; U.S. Food and Drug Administration 2004), there are several . Atropine eye drops are indicated for juvenile myopia progression, cycloplegia, amblyopia, and strabismus. According to the package insert, 10 mg/mL atropine eye drops must be diluted for pediatric patients to prevent systemic adverse effects. . Sterility assay. The sterility test method was validated using a method adapted from the Japanese .
sterility of eye drops
ophthalmic products for sterility
Topper de Bolo Fiona Persoarte Lembrancinhas R$ 11,90 2x R$ 5,95 sem juros; Topo de Bolo Fiona Natalia Guimarães Design R$ 25,00 5x R$ 5,64; Topo de Bolo Princesa .
sterility test for eye drops|microbiology of eye drops